Given:
Number of moles, n = 1 mol
Temperature, T = 0°C
Pressure, P = 1 atm
Let's find the volume.
To find the volume apply the ideal gas law:

Where:
• P is the pressure = 1 atm
,
• T is the temperature in kelvin = 0°C + 273 = 273 K
,
• n is the number of moles = 1 mol
,
• R is the universal gas constant = 0.08206 L.atm/mol.K
,
• V is the Volume.
Rewrite the formula for V:
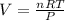
Substitute the values into the formula and solve for V:
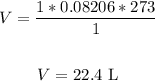
Therefore, the volume is 22.4 L
ANSWER:
22.4 L