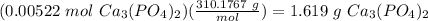First, use the molar mass of calcium sulfide (72.143 g/mol) to find the number of moles of CaS in 1.13 grams:
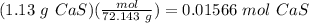
Now the equation for this reaction is
Li3PO4 + CaS --> Ca3(PO4)2 + Li2S
but the BALANCED equation is
2Li3PO4 + 3CaS --> Ca3(PO4)2 + 3Li2S
because in this, there is an equal number of every atom. In the balanced equation, there are 3 moles of CaS for every one mole of Ca3(PO4)2 created, so we change it like this:
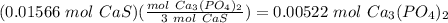
The molar mass of Ca3(PO4)2 is 310.1767 g/mol: