Answer:
12.18g
Step-by-step explanation:
You start with a mass of 20.3g of NH₄NO₃
We need to determine from this compound the mass of oxygen that is therein.
First, find the molar mass of the compound;
NH₄NO₃ = 14 + 4(1) + 14 + 3(16) = 80g/mol
So;
Mass of oxygen =
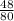 x 20.3 = 12.18g
x 20.3 = 12.18g
Since in a chemical reaction, mass is always conserved, 12.18g of oxygen is produced.