Answer:
The final pressure of the sample is 292.81 torr.
Step-by-step explanation:
The given information from the exercise is:
- Initial pressure (P1): 758.3torr
- Initial volume (V1): 50.7mL
- Final volume (V2): 131.3mL
We can clculate the final pressure (V2) of the neon sample, using the Boyle's law formula:
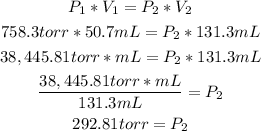
So, the final pressure of the sample is 292.81 torr.