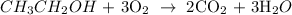Answer:
Step-by-step explanation:
Here, we want to write the correct equation for the combustion of ethanol
When organic compound burns in an unlimited supply of oxygen, what is produced is carbon (iv) oxide and water
We have the balaced chemical reaction as shown below: