Answer: 21.6%
Explanation: From given information, mass of hydrate is 5.1 g. It loses water on heating. So, mass of water in the hydrate is 1.1 g.
(4.0 g is the mass of the unhydrated salt which is not of our use to solve this problem.)
Percent by mass of water in the original hydrate is the mass of water divided by the mass of hydrate and multiplied by 100.
water percentage in hydrate =
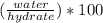
water percentage in hydrate =
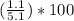
water percentage in hydrate = 21.6%
So, choice C) 21.6% is correct.