Answer
b. 5
Step-by-step explanation
The moles of CaCO3 present in 500 grams of CCO3 can be calculated using the mole formula.
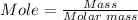
Molar mass of CaCO3 100 g/mol
Putting mass = 500 g and molar mass = 100 g/mol into the mole formula, we have,
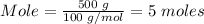
The moles of CaCO3 resent in 500 grams of CacCO3 = 5 moles