Step 1 - Understanding the equilibrium constant
For a chemical reaction:
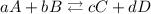
The equilibrium constant would be:
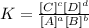
We need to be careful because pure liquids and solids do not enter the equilibrium constant.
Step 2 - Finding the equilibrium constant for the given reaction
For this reaction:
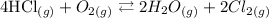
We can see that all substances are in the gaseous state. Therefore, all substances will enter the equilibrium constant. The equilibrium constant in this case will be:
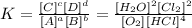
The correct answer is thus alternative e).