Answer:
1)
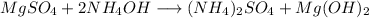
2) Double displacement reaction
Step-by-step explanation:
1) Balancing the equation:
The reaction:
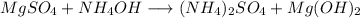
In a first look we see that the amonium cations are unbalanced so:
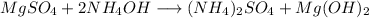
Checking element by element we can see that the equation is balanced
2) In this reaction, the amonium discplaces the Mg to form the amonium sulfate. This is a displacement reaction.
Given that it also forms another product (
 ) it's categorized as a double displacement reaction.
) it's categorized as a double displacement reaction.