Step-by-step explanation:
Wavelength in an emission spectrum,

The energy of an electron is given by :
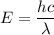
Where
h is the Planck's constant
c is the speed of light
For 435 nm, the energy of the electron will be :
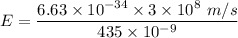
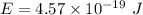
We know that
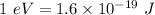
So,
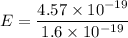
So, E = 2.86 eV
The energy of the electron dropping from one energy level is 2.86 eV. We know that,
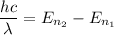
From the given energy levels :
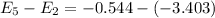

So, the transition must be from E₅ to E₂. Hence, this is the required solution.