Answer: The mass of sodium making up the given amount of sodium chloride will be 59.01 g.
Step-by-step explanation:
We are given:
Mass of sodium chloride is 100 g which is made from 39.34 g of sodium and 60.66 g of chlorine.
So, to calculate the mass of sodium which makes up 150 grams of sodium chloride, we use unitary method.
Mass of sodium required will be =
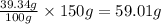
Hence, the mass of sodium making up the given amount of sodium chloride will be 59.01 g.