Answer:
Step-by-step explanation:
The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time. Let's see the following reaction:
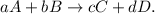
The rate can be measured in terms of either reactant (A or B) or either product (C or D), like this:
![rate=-(1)/(a)\cdot(\Delta\lbrack A])/(\Delta t)=-(1)/(b)\cdot(\Delta\lbrack B])/(\Delta t)=(1)/(c)\cdot(\Delta\lbrack C])/(\Delta t)=(1)/(d)\cdot(\Delta\lbrack D])/(\Delta t).](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/87zomgvyka9lqulkgpcr.png)
The problem is asking for the rate of the reaction in terms of the change of nitrogen dioxide (NO2) concentration, so we can apply the formula of rate.
As NO2 are in the reactants, the rate is negative because it is consuming and the coefficient which is 14 is in the denominator:
![rate=-(1)/(14)\cdot(\Delta\lbrack NO_2])/(\Delta t).](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/td1mnhvabwfn9wuhz7ds.png)
The answer would be that the rate of NO2 concentration in the reaction is -1/14*(Δ[NO2])/Δt).