Answer: Option (a) is the correct answer.
Step-by-step explanation:
A base is a substance that gives hydroxide ions (
 ) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution.
) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution.
On the other hand, an acid is a substance that gives hydrogen ions (
 ) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.
) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.
For example,
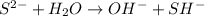
Since, sodium sulfide (
 ) dissociates to give hydroxide ions.
) dissociates to give hydroxide ions.
Hence, we can conclude that
 is a base because it ionizes to release
is a base because it ionizes to release
 .
.