Answer: The correct answer is Option C.
Step-by-step explanation:
Elements are arranged in 7 periods and 18 groups. Every period ends with elements present in Group 18.
The electronic configuration for Group 18 is

where, n = number of period.
The elements present in group 18 are known as noble gas or inert gas.
For Example: Argon is present in 3rd period. The electronic configuration ofr this element is
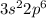 . We know that for n = 3, 3d orbital is also present but it is not filled in argon element.
. We know that for n = 3, 3d orbital is also present but it is not filled in argon element.
Thus, the last element of any period will show the properties of inert gases.
Hence, the correct answer is Option C.