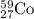Answer: The equations are given below.
Step-by-step explanation:
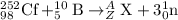
To calculate A:
Total mass on reactant side = total mass on product side
252 + 10 = A + 3
A = 259
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
98 + 5 = Z + 0
Z = 103
The isotopic symbol of lawrencium is
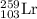
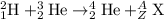
To calculate A:
Total mass on reactant side = total mass on product side
2 + 3 = A + 4
A = 1
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
1 + 2 = Z + 2
Z = 1
The isotopic symbol of hydrogen is
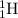
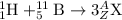
To calculate A:
Total mass on reactant side = total mass on product side
1 + 11 = A
A = 12
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
1 + 5 = Z
Z = 6
The isotopic symbol of helium is
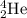
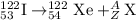
To calculate A:
Total mass on reactant side = total mass on product side
122 = A
A = 122
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
53 = 54 + Z
Z = -1
The isotopic symbol of electron is
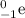

To calculate A:
Total mass on reactant side = total mass on product side
59 + 0 = A
A = 59
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
26 = -1 + Z
Z = 27
The isotopic symbol of Cobalt is