Answer: Option (a) is the correct answer.
Step-by-step explanation:
When a neutral atom loses an electron then it develops a positive charge and results into the formation of a cation.
When only one electron is lost by the atom then it is called a monoatomic cation.
When two electrons are lost by the atom then it is called a diatomic cation.
For example, Cs is an alkali metal and when it loses it one valence electrons then it changes into
 ion.
ion.
Whereas
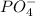 ,
,
 are all ions of compound since they contain more than one element.
are all ions of compound since they contain more than one element.
And, there is no charge on Br atom. This means that it is neutral in nature.
Thus, we can conclude that out of the given options
 is a monatomic cation.
is a monatomic cation.