Answer:
The average kinetic energy of the gas is 4.678 kJ/mol.
Step-by-step explanation:
Average kinetic energy of the gas id defined as measure of an average kinetic energy possessed by each particle of the gas.
It is given as:
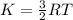 (Average kinetic energy per mole)
(Average kinetic energy per mole)
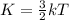 (Average kinetic energy per molecule)
(Average kinetic energy per molecule)
R = Universals gas constant
T = temperature of the gas
k = Boltzmann constant =

The average kinetic energy of 1 mole of gas at 102°C:
T = 102°C = 375.15 K[/tex]
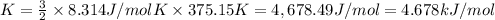
The average kinetic energy of the gas is 4.678 kJ/mol.