Hello!
Find the empirical formula of a compound which contains 54.93% potassium, 38.73% boron and 6.34% hydrogen.
A. KBH
B. KB2H4
C. KB3H9
D. K2B5H9
- We have the following data:
Potassium (K) ≈ 39 a.m.u (g/mol)
Boron (B) ≈ 11 a.m.u (g/mol)
Hydrogen (H) ≈ 1 a.m.u (g/mol)
- We use the amount in grams (mass ratio) based on the composition of the elements, see: (in 100g solution)
K: 54.93 % = 54.93 g
B: 38.73 % = 38.73 g
H: 6.34 % = 6.34 g
- The values (in g) will be converted into quantity of substance (number of mols), dividing by molecular weight (g / mol) each of the values, we will see:
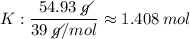
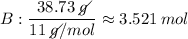
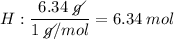
- We realize that the values found above are not integers, so we divide these values by the smallest of them, so that the proportion does not change, let us see:
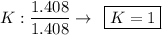
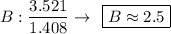
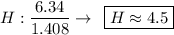
convert number of atomic radio into whole number
2 * (1 : 2.5 : 4.5)
= 2 : 5 : 9 ← whole number of atomic radio
K = 2
B = 5
H = 9
- Thus, the minimum or empirical formula found for the compound will be:
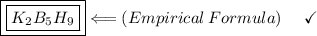
Answer:
D. K2B5H9
________________________
