Step-by-step explanation
A molecule will always have a neutral charge, so the oxidation states of the elements must be such that the sum of the oxidation states is zero.
Now, the oxidation state of oxygen is -2, because there are 4 oxygen atoms so far we have an oxidation state of -8.
The oxidation state of potassium is always +1, and we have a potassium atom in the molecule, so we have a total oxidation state of:
-8+1=-7.
For the molecule to be neutral, the oxidation state that we need is +7, and since there is only one atom of magnesium we will have to:
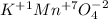
The oxidation number of manganese in KMnO4 is+7
Answer: (1) +7