Step-by-step explanation:
When an acid reacts with a base then it results in the formation of salt and water.
 is an acid and
is an acid and
 is a base thus, when we dissolve ammonium hydroxide in nitric acid then it results in the formation of ammonium nitrate and water.
is a base thus, when we dissolve ammonium hydroxide in nitric acid then it results in the formation of ammonium nitrate and water.
The reaction is as follows.
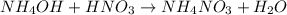
Hence, there will be formation of ammonium nitrate
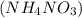 salt.
salt.