The formula that describes radioactive decay over time, is the following:
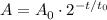
Where A is the amount of a radioactive element after a time t, if t_0 is the half-life of the given element, for an initial sample A_0.
Solve for A_0, and substitute A=1.3g, t=93.2 and t_0=8.07 to find the initial amount of iodine-131 in the original sample:
![undefined]()