Step-by-step explanation:
First, let's write the chemical reaction:
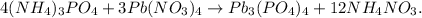
Now, let's find what is the limiting reactant. Let's find how many moles of Pb3(PO4)4 are being produced by each reactant.
You can see that 4 moles of (NH4)3PO4 reacted produces 1 mol Pb3(PO4)4, so 5.20 moles of (NH4)3PO4 will produce:
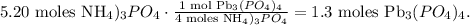
And now, let's see how many moles of Pb3(PO4)4 are being produced by 4.35 moles of Pb(NO3)4. In the chemical reaction you can see that 3 moles of Pb(NO3)4 reacted produces 1 mol of Pb3(PO4)4:
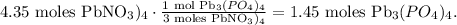
You can realize that we have an excess of Pb(NO3)4 because the limit is imposed by (NH4)3PO4. So let's find how many moles of Pb(NO3)4 are required to produce 1.3 moles of Pb(NO3)4, like this:
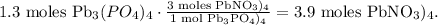
This means that we have an excess of 0.45 moles of Pb(NO3)4 (4.35 moles - 3.9 moles = 0.45 moles).
Now, let's find the number of moles that are being produced of NH4NO3 by the limiting reactant which is (NH4)3PO4. 4 moles of (NH4)3PO4 reacted produces 12 moles of NH4NO3:
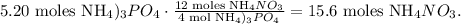
If we follow the same steps that we followed before, we're going to see that the excess (Pb(NO3)4) will produce 17.4 moles of NH4NO3, and actually, to form 15.6 moles of NH4NO3 we need 3.9 moles of Pb(NO3)4 as we calculated before.
Answer:
And finally, we can complete the BCA table: