Answer: The correct answer is Option B.
Step-by-step explanation:
Double displacement reaction is defined as the reaction in which exchange of ions takes place.

For the given options:
The chemical equation follows:

This is a type of combustion reaction because an organic compound reacts with oxygen gas to produce carbon dioxide gas and water molecule
The chemical equation follows:
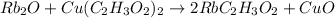
This is a type of double displacement reaction.
The chemical equation follows:
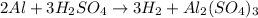
This is a type of single displacement reaction because a more reactive metal (aluminium) is replacing a less reactive metal (hydrogen).
The chemical equation follows:

This is a type of decomposition reaction because a single large compound is getting broken down to smaller substances.
Hence, the correct answer is Option B.