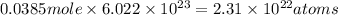Step-by-step explanation:
Given mass of sugar = 1.202 g
Molar mass of the sugar ,
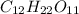 = 342.29 g/mol
= 342.29 g/mol
a) Number of moles of sugar
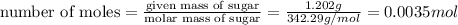
Number of moles of sugar in 1.202 grams is 0.0035 moles
b)Moles of each element in
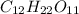
In a molecular formula of sugar there are 12 carbon, 22 hydrogen and 11 oxygen atoms
Moles of an element in molecule =
number of atoms of that element in a molecular formula × number of moles of compound
Moles of carbon = 12 × 0.0035 = 0.042 moles
Similarly
Moles of hydrogen = 22 × 0.0035 = 0.077 moles
Moles of oxygen =11 × 0.0035 = 0.0385 moles
c) Number of atoms of each type in
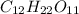
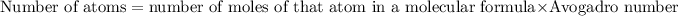
Number of carbon atoms =
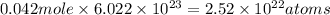
Number of hydrogen =
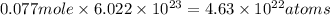
Number of oxygen atoms =