Answer:
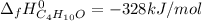
Step-by-step explanation:
Hello,
Considering the specified chemical reaction, one writes the expression to compute this reaction's heat as shown below:
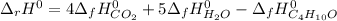
Now, solving for the standard enthalpy of formation of liquid butanol, one obtains:
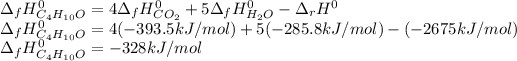
Best regards.