The formula of mass percent is the following:
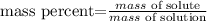
where mass of solute correspond to the mass of NaCl and mass of solution correspond to the sum of NaCl and water mass.
We need to find the mass of water using its volume and density:
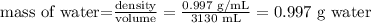
And we replace it in the first formula like this:
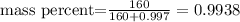
We obtain the percent multiplying the result by 100, so the answer is 99.38%