Answer:
A) 5.83*10^-2
Step-by-step explanation:
The information given from the exercise is:
- Kp value: 1.45x10^-5
- Temperature (T): 500°C
- Chemical reaction
We can calculate the value of Kc by replacing the values of R (gas constant: 0.082 atm*L/mol*K), temperature (T) and Δn in the Kp formula:
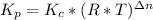
In this formula, Δn is the result of the difference between the products coefficients and the reactants ccoefficents:
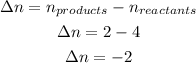
It is important to convert the unit of temperature from °C to K:
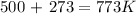
Now we can replace the values:
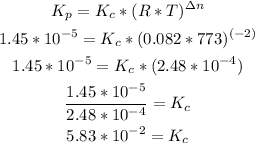
So, the value of Kc is 5.83*10^-2.