Answer:
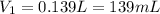
Step-by-step explanation:
Hello!
In this case, since the dilution processes are characterized by the decrease of the original stock solution by holding the moles constant and therefore modify the volume, for the described dilution we can write:
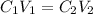
Whereas we are asked to compute the volume of the original solution; thus, we can solve for it as shown below:
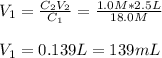
Best regards!