Answer:
1.46 grams.
Explanations:
Given the following parameters from the question.
Molarity of NaCl = 0.5M
Volume of NaCl = 50ml = 0.05L
Determine the moles of NaCl
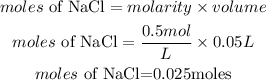
Calculate the mass of NaCl needed
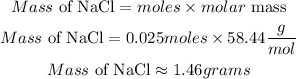
Hence the number of grams needed to make 50 ml of a 0.5M NaCl solution to the nearest 0.01 is 1.46 grams.