Answer
0.8886 mol
Step-by-step explanation
Given:
Mass of Ag₃N = 300 grams
What to find:
The number of moles present in 300 grams of Ag₃N.
Step-by=step solution:
From the Periodic Table; the 1 mole of Ag₃N has 337.61 g/mol
Therefore, the number of moles present in 300 grams Ag₃N can be calculated using the mole formula below:
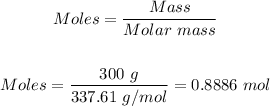
The number of moles present in 300 grams of Ag₃N is 0.8886 mol.