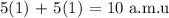Answer:
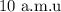
Step-by-step explanation:
Here, we want to get the mass of the given atom
The atomic mass is simply the sum of the mass of the protons and neutrons
From the nucleus, we can see that we have 5 protons and 5 neutrons
Although they have different charges, they have roughly the same mass
The mass of each is approximately equal to 1
Thus, we have the mass of the atom as;