Answer:
![\begin{gathered} a)\text{ }([H_3O^+][Ac^-])/([HA_c]) \\ b)\text{ HAc - acid} \\ H_2O-base \\ Ac^--conjugate\text{ base} \\ H_3O^+-conjugate\text{ acid} \\ c)\text{ H}_2O \\ d)\text{ Ac}^{-\text{ }}is\text{ stronger since k > 0} \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/9nbs4tmv71anjyjj34no.png)
Explanations:
Given the equilibrium expression shown below;
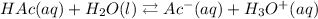
a) The equilibrium expression for the reaction is given as:
![\begin{gathered} k_a=([H_3O^(^+)][Ac^-])/([H_2O][HAc]) \\ Since\text{ }[H_2O]-1,\text{ hence;} \\ k_a=([H_3O^+][Ac^-])/([HA_c]) \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/3v8eb9l58vb0su7uvvr2.png)
The equilibrium constant we are using will be Ka.
b) The compound that is given off a proton is an acid while the compound accepting a proton is a base. From the given equation HAc acts as an acid while H2O acts as a base. Ac- is the conjugate base while H3O+ is the conjugate acid.
c) The base that is competing for a proton is H2O (water)
d) Since a lower value of k shows a stronger base, hence the conjugate base hence H2O will be a stronger base than that of Ac. Recall that if k < 1, the reverse reaction is favoured and the forward reaction does not proceed to a great extent showing that H2O will be the stronger base.