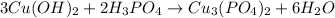Answer:
3, 2, 1, 6
Step-by-step explanation:
The unbalanced combustion reaction is shown below as:-
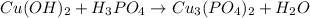
On the left hand side,
There are 1 copper atom and 1 phosphorus atom
On the right hand side,
There are 3 copper atoms and 2 phosphorus atoms
Thus,
Left side,
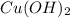 must be multiplied by 3 and
must be multiplied by 3 and
 by 2 so to balance copper phosphorus atoms.
by 2 so to balance copper phosphorus atoms.
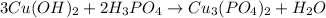
Left side, 12 H, so
 must be multiplied by 6.
must be multiplied by 6.
Thus, the balanced reaction is:-