Answer:
Atomic number = 15
Element = Phosphorous.
Step-by-step explanation:
Hello,
In this case, taking into account that the neutrons are obtained through the following equation:
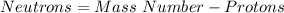
In such a way, the protons match with the element's atomic number and therefore its identity, thus:
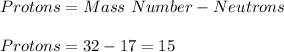
Therefore, the element is phosphorous.
Best regards.