Step-by-step explanation:
The highest occupied energy sub-level is defined as number of electrons present in the outermost energy level of an atom.
For example, for alkali metals general electronic configuration is
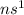 .
.
This means that in the highest occupied energy sub-level of an alkali metal there will be only one electron.
Whereas for alkaline earth metals general electronic configuration is
 . This means there are 2 electrons present in the highest occupied energy sub-level of an alkaline earth metal.
. This means there are 2 electrons present in the highest occupied energy sub-level of an alkaline earth metal.
Thus, we can conclude that atom that contains only one electron in the highest occupied energy sublevel are alkali metals.