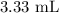Answer:
The quantity of a 30% solution you would need to make 50 mL of a 2% solution is;
Step-by-step explanation:
Let x represent the quantity of a 30% solution you would need to make 50 mL of a 2% solution.
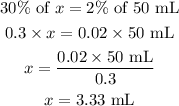
Therefore, the quantity of a 30% solution you would need to make 50 mL of a 2% solution is;