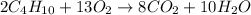Answer : The balanced chemical reaction will be,
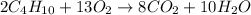
Explanation :
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
If the amount of atoms of each type on the left and right sides of a reaction differs then to balance the equation by adding coefficient in the front of the elements or molecule or compound in the chemical equation.
The coefficient tell us about that how many molecules or atoms present in the chemical equation.
The given chemical reaction is,

This reaction is an unbalanced chemical reaction because in this reaction number of carbon, hydrogen and oxygen atoms are not balanced.
In order to balance the chemical equation, the coefficient '2' is put before the
 , coefficient '13' is put before the
, coefficient '13' is put before the
 , coefficient '8' is put before the
, coefficient '8' is put before the
 and coefficient '10' is put before the
and coefficient '10' is put before the
 and we get the balanced chemical equation.
and we get the balanced chemical equation.
The balanced chemical reaction will be,