Answer : The number of moles of carbon in 2.0 mole of caffeine are, 16 moles
Solution :
The formula of caffeine is,
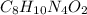
As we know that, 1 mole of caffeine contains 8 moles of carbon atom, 10 moles of hydrogen atoms, 4 moles of nitrogen atoms and 2 moles of oxygen atoms.
According to the question,
As, 1 mole of caffeine contains 8 moles of carbon atoms
So, 2 moles of caffeine contains
 moles of carbon atoms
moles of carbon atoms
Therefore, the number of moles of carbon in 2.0 mole of caffeine are, 16 moles