Answer
The specific heat capacity of quartz (to 2 significant digits) according to the experiment is 0.74 J/g⁰C
Step-by-step explanation
Given parameters:
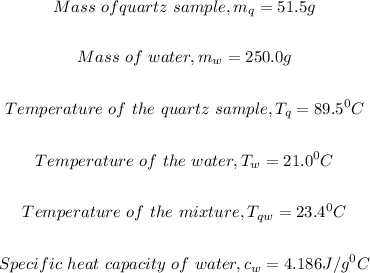
What to find:
The specific heat capacity of quartz (Cq) according to this experiment.
Step-by-step solution:
The specific heat capacity of quartz (Cq) according to this experiment can be calculated using the formula for heat lost = heat gained.
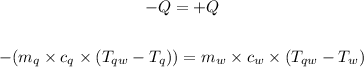
The temperature of the quartz is higher than that of the water, so the quartz will give off energy in form of heat to the water.
Putting the values of the given parameters into the formula above, we have:

Thus, the specific heat capacity of quartz (to 2 significant digits) according to the experiment is 0.74 J/g⁰C