Answer: The metallic oxide formed will be

Step-by-step explanation:
We are given a metallic oxide, having general chemical formula

We are given:
Mass of metallic oxide = 1.187 g
Mass of metal = 1.054 g
Mass of oxygen = 1.187 - 1.054 = 0.133 g
To calculate the number of moles, we use the equation:
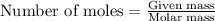 .......(1)
.......(1)
Given mass of oxygen = 0.133 g
Molar mass of oxygen = 16 g/mol
Putting values equation 1, we get:

Number of moles of metal in the oxide is twice than the number of moles of oxygen
Number of moles of metal =
 moles
moles
Now, calculating the molar mass of metal by using equation 1, we get:
Moles of metal = 0.0166 moles
Mass of metal = 1.054 g
Putting values in equation 1, we get:

The metal having 63.49 g/mol as molar mass is copper
Hence, the metallic oxide formed will be
