ANSWER
The wavelength of the photon is 0.0053nm
Explanation:
Given data
Frequency of the photon = 5.66 x 10^-4 Hz
Let x represents the wavelength of the photon
To determine the wavelength of the photon, we need to apply the below formula

Recall that, the speed of light is 3 x 10^8 m/s
The next process is to substitute the values into the above formula
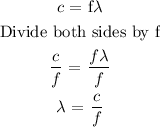
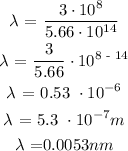
Therefore, the wavelength of the photon is 0.0053nm