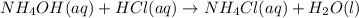Answer:
Neutralization reaction occurs between aqueous ammonia solution and an acid.
Step-by-step explanation:
Reaction that takes place between aqueous ammonia and acid is neutralization reaction.
A neutralization reaction is defined as chemical reaction between acid and base which results in formation of salt and water as a product.
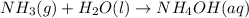
So, when HCl reacts with liquid ammonia it gives aqueous solution of ammonium chloride and water as a predict.