Answer:
20.375 moles of oxygen are necessary to completely react with propane to produce 16.3 moles of water.
Step-by-step explanation:
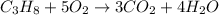
Mole sof water formed = 16.3 moles
According to reaction , 4 moles of water are obtained from 5 moles of oxygen.
Then 16.3 moles of oxygen will be obtained from:
 of oxygen
of oxygen
20.375 moles of oxygen are necessary to completely react with propane.