Step-by-step explanation:
A molecule which contains three bond pairs but no lone pair of electrons will have a trigonal planar geometry.
Hybridization of such a geometry is
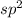 .
.
For example,
 is a trigonal planar molecule.
is a trigonal planar molecule.
As, hybridization of
 will be calculated as follows.
will be calculated as follows.
Hybridization =
![(1)/(2)[V+N-C+A]](https://img.qammunity.org/2017/formulas/chemistry/high-school/mcmqr7761agnmufgz2fbdxrkvtui8e4mzk.png)
where,
V = number of valence electrons present in central atom
N = number of monovalent atoms bonded to central atom
C = charge of cation
A = charge of anion
Hybridization =
![(1)/(2)[V+N-C+A]](https://img.qammunity.org/2017/formulas/chemistry/high-school/mcmqr7761agnmufgz2fbdxrkvtui8e4mzk.png)
=
![(1)/(2)[3 + 3]](https://img.qammunity.org/2017/formulas/chemistry/high-school/nptgtff9yn1daisepblmcht8j46a9s2vdm.png)
= 3
So, the hybridization of
 will be
will be
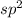 . As there are 3 bond pairs and no lone pair of electrons. Hence, the geometry of
. As there are 3 bond pairs and no lone pair of electrons. Hence, the geometry of
 is trigonal planar.
is trigonal planar.
Thus, we can conclude that according to the VSEPR theory, the shape of a molecule that has a central atom bound to three other atoms with no lone pairs of electrons is trigonal planar.