Given:
• Planck's constant, h = 6.626×10⁻³⁴ J⋅s
,
• Speed of light, c = 2.99×10⁸ m/s
,
• Energy = 4.80×10⁻¹⁹ J
Let's find the wavelength of the photon.
Apply the formula for photon energy:
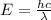
Where:
λ is the wavelength.
Rewrite the formula for λ, plug in the values and solve.
We have:
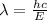
Thus, we have:
![\begin{gathered} \lambda=(6.626*10^(-34)*2.99*10^8)/(4.80*10^(-19)) \\ \\ \lambda=\frac{1.981174\operatorname{*}10^(-25)}{4.80\operatorname{*}10^(-19)} \\ \\ \lambda=4.13*10^(-7)\text{ m} \end{gathered}]()
Therefore, the wavelength of the photon is 4.13 x 10⁻⁷ meters.
• ANSWER:
4.13 x 10⁻⁷ m.