Step-by-step explanation:
To calculate the number of moles, we use the equation:
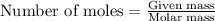 ......(1)
......(1)
Given mass of water = 4.50 g
Molar mass of water = 18 g/mol
Putting values in equation 1, we get:

Hence, the moles of given amount of water is 0.25 moles.
- For 2: 471.6 g of barium hydroxide
Given mass of barium hydroxide = 471.6 g
Molar mass of barium hydroxide = 171.34 g/mol
Putting values in equation 1, we get:

Hence, the moles of given amount of barium hydroxide is 2.75 moles.
- For 3: 129.68 g of iron phosphate
Given mass of iron phosphate = 129.68 g
Molar mass of iron phosphate = 150.82 g/mol
Putting values in equation 1, we get:

Hence, the moles of given amount of iron phosphate is 0.86 moles.