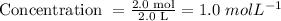Answer
1.0 mol/L
Step-by-step explanation
Given:
The number of moles of solute = 2.0 mol
The volume of solvent = 2.0 L
What to find:
The concentration of the solution.
Step-by-step solution:
The resulting concentration of the solution can be calculated using the formula:
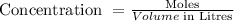
To get the concentration, substitute the given data into the formula.