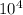Answer:
The standard form of ( 6.31×
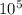 ) + (2.6 ×
) + (2.6 ×
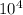 ) = 65.7 ×
) = 65.7 ×
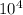
Step-by-step explanation:
As given,
( 6.31×
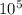 ) + (2.6 ×
) + (2.6 ×
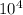 ) = ( 6.31×
) = ( 6.31×
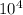 ×10 ) + (2.6 ×
×10 ) + (2.6 ×
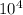 )
)
=
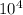 ( 6.31×10 + 2.6 )
( 6.31×10 + 2.6 )
=
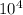 ( 63.1 + 2.6 )
( 63.1 + 2.6 )
=
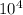 ( 65.7 )
( 65.7 )
= 65.7 ×
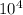
So, the standard form of ( 6.31×
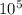 ) + (2.6 ×
) + (2.6 ×
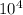 ) = 65.7 ×
) = 65.7 ×