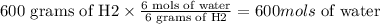According to the reaction we need 6 mols of water to produce 3 mols of H2 gas
but since they are asking about the mass we need to change these 3 mols to mass, for that we multiply the number of mols (3) by the molar mass of H2 (2):
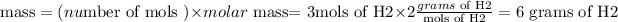
So after this calculation we know that 6 grams of H2 are produced each 6 mols of water are consumed using this as a conversion factor we can write the following equation: