Answer: The pressure of the gas is 19 atm.
Explanation: By using ideal gas equation, which is:
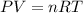
Given conditions are:
T = 20°C = (273 + 20)K = 293K
V = 5 gallon = ( 5 × 3.97)L = 18.95L
n = 15 moles
 (Gas Constant)
(Gas Constant)
P = ? atm
Putting all the values in above equation, we get
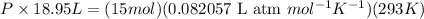
P = 19 atm
Therefore, the pressure of the gas is found to be 19 atm.